Human Papilloma Virüsü (HPV) rahim ağzı kanserine (cervical cancer) neden olmaktadır(1,2). HPV’ nin 200′ den fazla çeşidi (genotypes) vardır(3). HPV nin genetik yapısında oluşan değişiklikler (mutasyonlar) her geçen gün yeni çeşitlerin ortaya çıkmasına neden olmaktadır. (3,4,5). Bunlardan şimdilik yaklaşık 20 tanesinin rahim ağzı kanserine neden olduğu bilinmektedir(1,3).
HPV enfeksiyonu genellikle akıntı, kanama ve ağrı gibi şikayetler yapmamaktadır. Gözle görülemeyen, ancak mikroskop ile görülebilen hücre içinde bazı değişikliklere (Squamous Intraepithelial Lesion) (SIL) neden olmaktadır(6,7,8,9,10,11,12). Bu hücresel değişiklikler ancak pap smear testi ile görülebilmektedir. HPV DNA testinde HPV enfeksiyonu tespit edilenlerin (pozitif) bir kısmında ise erken teşhis nedeniyle pap smear testinde henüz hücreye zarar verecek kadar süre geçmediği için hücresel değişiklik (SIL) görülmemektedir(13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28). HPV DNA testi ve Pap smear testi birlikte yapıldığında bir kişide HPV enfeksiyonu olduğu halde bu testlerin yanlışlıkla yok deme ihtimali de, bir kişide HPV enfeksiyonu olmadığı halde yanlışlıkla var deme ihtimali de oldukça düşüktür(29,30,31,32,33,34,35,36,37,38,39,40,41,42).
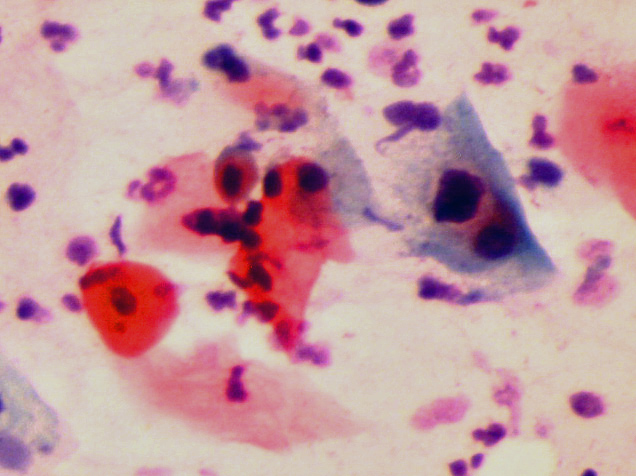
Pap smear testi ve HPV DNA testi için muayene sırasında rahim ağzından, rahim ağzı kanserinin en çok görüldüğü bölgeden (transformation zone) çubuk şeklinde, ince bir fırça yardımıyla akıntı örneği alınır(43,44,45). Bu akıntı örneğinde görülen hücrelerde HPV DNA’ sının varlığını tespit etmek için HPV DNA PCR testi yapılır, pap smear testinde de akıntı örneğindeki rahim ağzı hücreleri mikroskop ile incelenerek HPV enfeksiyonunun neden olduğu değişiklikler görülür. (8,11,12,31,31,32,43,44,45,46,48,49,50,51).
Rahim ağzında görülen HPV enfeksiyonlarının büyük çoğunluğu (%90) normal bağışıklığa sahip olan kişilerde yaklaşık 1 yıl içinde, daha küçük bir kısmında da 2 yıl içinde kendiliğinden vücuttan temizlenmektedir (clearance)(23,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69). Rahim ağzında yaptıkları hücresel değişikliklerin (SIL) büyük bir kısmı da (%90) 2 yıla kadar iyileşmektedir(7,23,52,53,54,55,56,62,63,65,70,71,72,73,74,75). Vücuttan temizlenmeyenlerin (persistence) büyük çoğunluğunu rahim ağzı kanserine neden olan HPV çeşitleri oluşturmaktadır(53,55,57,68,70). Rahim ağzında ortaya çıkan ileri derecede hücresel değişikliklere (Cervical Intraepithelial Neoplasia) (CIN+2) genellikle 3 yıl içinde vücuttan temizlenmeyen HPV çeşitleri neden olmaktadir(7,43,48,58,59,65,66,67,69,70,71,72,76). Rahim ağzı kanserlerinin büyük bir kısmına 2 yıl içinde vücuttan temizlenmeyen HPV-16 ve HPV-18 çeşitleri neden olmaktadır(55,56,63,69). Demek ki burada doğru olan kendiliğinden iyileşmeyen, rahim ağzı kanseri riski taşıyan kişinin tespit edilerek onun HPV DNA testi ve pap smear testi ile takibinin ve tedavisinin yapılmasıdır(1,34,35,38,39,48,77). Rahim ağzı kanseri ve ona bağlı gelişen ölümler çoğunlukla Pap smear testi ve HPV DNA testi yaptırmayanlarda görülmektedir(49,77,78,79,80).
Human Papilloma Virüs (HPV) enfeksiyonunun neden olduğu rahim ağzı (serviks) kanserinin polikistik over sendromu ile bir ilişkisi yoktur. Rahim ağzı kanseri polikistik over sendromu olan kadınlarda diğer kadınlarda görüldüğünden daha fazla görülmemektedir.
Kaynaklar
1-Are 20 human papillomavirus types causing cervical cancer? Arbyn M, Tommasino M, Depuydt C, Dillner J. J Pathol. 2014 Dec;234(4):431-5. 2-Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Walboomers JM,Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. J Pathol. 1999 Sep;189(1):12-9. 3-International standardization and classification of human papillomavirus types. Bzhalava D, Eklund C, Dillner J. Virology. 2015 Feb;476:341-344. 4-Naturally occurring capsid protein variants L1 of human papillomavirus genotype 16 in Morocco. El-Aliani A, Alaoui MAE, Chaoui I, Ennaji MM, Attaleb M, Mzibri ME. Bioinformation. 2017 Aug 31;13(8):241-248. 5-Abundance of HPV L1 Intra-Genotype Variants With Capsid Epitopic Modifications Found Within Low- and High-Grade Pap Smears With Potential Implications for Vaccinology. Shen-Gunther J, Cai H, Zhang H, Wang Y. Front Genet. 2019 May 24;10:489. 6-A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen TE, Fortenberry JD. J Infect Dis. 2005 Jan 15;191(2):182-92. 7-Development and duration of human papillomavirus lesions, after initial infection. Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. J Infect Dis. 2005 Mar 1;191(5):731-8. 8-The 2001 Bethesda System: terminology for reporting results of cervical cytology. Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, Young N; Forum Group Members; Bethesda 2001 Workshop. JAMA. 2002 Apr 24;287(16):2114-9. 9-Regression of low-grade squamous intra-epithelial lesions in young women. Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, Miller S, Canjura-Clayton KL, Farhat S, Broering JM, Darragh TM. Lancet. 2004 Nov 6-12;364(9446):1678-83. 10-A study of 10,296 pediatric and adolescent Papanicolaou smear diagnoses in northern New England. Mount SL, Papillo JL. Pediatrics. 1999 Mar;103(3):539-45. 11-Endocervical and metaplastic cells: comparison of endocervical and metaplastic cell number in Papanicolaou smears with and without squamous intraepithelial lesion. Narges Izadi Mood, Hadi Mozaffari Miandoab. Acta Cytol. Mar-Apr 2006;50(2):178-80. 13-The significance of squamous metaplasia in the development of low grade squamous intraepithelial lesions in young women. Moscicki AB, Burt VG, Kanowitz S, Darragh T, Shiboski S. Cancer. 1999 Mar 1;85(5):1139-44. 14-Human papillomavirus prevalence in women attending routine cervical screening in South Wales, UK: a cross-sectional study. S Hibbitts, J Jones, N Powell, N Dallimore, J McRea, H Beer, A Tristram, H Fielder, A N Fiander. Br J Cancer. 2008 Dec 2;99(11):1929-33. 15-Prevalence of high-risk human papillomavirus (HR-HPV) types 16 and 18 in healthy women with cytologically negative Pap smear. Raksha Arora, Arunaz Kumar, Bhupesh K Prusty, Uma Kailash, Swaraj Batra, Bhudev C Das. Eur J Obstet Gynecol Reprod Biol. 2005 Jul 1;121(1):104. 16-HPV prevalence among Mexican women with neoplastic and normal cervixes.M Torroella-Kouri, S Morsberger, A Carrillo, A Mohar, A Meneses, M Ibarra, R W Daniel, A M Ghaffari, G Solorza, K V Shah. Gynecol Oncol. 1998 Jul;70(1):115-20. 17-Genotype distribution and prevalence of human papillomavirus among women with cervical cytological abnormalities in Xinjiang, China. Wang J,Tang D, Wang J, Zhang Z, Chen Y, Wang K, Zhang X, Ma C. Hum Vaccin Immunother. 2019;15(7-8):1889-1896 18-Prevalence of HPV genotypes determined by PCR and DNA sequencing in cervical specimens from French women with or without abnormalities. C Pannier-Stockman, C Segard, S Bennamar, J Gondry, J-C Boulanger, H Sevestre, S Castelain, G Duverlie. J Clin Virol. 2008 Aug;42(4):353-60. 19-Human papillomavirus genotypes distribution by cervical cytologic status among women attending the General Hospital of Loandjili, Pointe-Noire, Southwest Congo (Brazzaville). Luc Magloire Anicet Boumba, Zineb Qmichou, Mustapha Mouallif, Mohammed Attaleb, Mohammed El Mzibri, Lahoucine Hilali, Moukassa Donatien, Moulay Mustapha Ennaji Affiliati. J Med Virol. 2015 Oct;87(10):1769-76. 20-The relationship of human papillomavirus (HPV) detection to pap smear classification of cervical-scraped cells in asymptomatic women in northeast Thailand. Ekalaksananan T, Pientong C, Kotimanusvanij D, Kongyingyoes B, Sriamporn S, Jintakanon D.J Obstet Gynaecol Res. 2001 Jun;27(3):117-24. 21-Frequency of human papillomavirus infection and genotype distribution among women with known cytological diagnosis in a Southern Italian region. Chironna M, Neve A, Sallustio A, De Robertis A, Quarto M, Germinario C, Lepera A, Cicinelli E, Carriero C, Pinto V, Miniello G, Borraccino V, Blasi N, Romano F, Noya E; HPV Study Group. J Prev Med Hyg. 2010 Dec;51(4):139-45. 22-The prevalence and genotype distribution of human papillomaviruses among women in Taizhou, China. Rongrong Jin, Hua Qian, Yongsheng Zhang, Donglan Yuan, Jingjing Bao, Huilin Zhou, Min Chen, Junxing Huang, Hong Yu. Medicine. 2019 Sep;98(39):e17293. 23-Persistence of type-specific human papillomavirus infection among Daqing City women in China with normal cytology: a pilot prospective study. Ni Li, Dong Hang, Lin Yang , Xiaoshuang Feng, et al. Oncotarget. 2017 Aug 11;8(46):81455-81461. 24-Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. Muñoz N, Méndez F,Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Muñoz A; Instituto Nacional de Cancerologia HPV Study Group. J Infect Dis. 2004 Dec 15;190(12):2077-87. 25-Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, Darragh T, Palefsky J. JAMA. 2001 Jun 20;285(23):2995-3002. 26-Prevalence and determinants of HPV infection among Colombian women with normal cytology. Molano M, Posso H, Weiderpass E, van den Brule AJ, Ronderos M, Franceschi S, Meijer CJ, Arslan A, Munoz N; HPV Study Group HPV Study. Br J Cancer. 2002 Jul 29;87(3):324-33. 27-Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Lancet Infect Dis. 2007 Jul;7(7):453-9. 28-Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, Bosch FX, Ferreccio C, Hieu NT, Lazcano-Ponce E, Matos E, Molano M, Qiao YL, Rajkumar R, Ronco G, de Sanjosé S, Shin HR, Sukvirach S, Thomas JO, Meijer CJ, Muñoz N. Int J Cancer. 2006 Dec 1;119(11):2677-84. 29-Benefits and harms of cervical screening from age 20 years compared with screening from age 25 years. Landy R, Birke H, Castanon A, Sasieni P. Br J Cancer. 2014 Apr 2;110(7):1841-6. 30-Cytology versus HPV testing for cervical cancer screening in the general population. Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, Schünemann H, Paraskevaidis E, Arbyn M. Cochrane Database Syst Rev. 2017 Aug 10;8(8):CD008587. 31-Pap smear adequacy: Is our understanding satisfactory…or limited? Birdsong GG.Diagn Cytopathol. 2001 Feb;24(2):79-81. 32-The significance of endocervical cells and metaplastic squamous cells in liquid-based cervical cytology. Leung KM, Lam M, Lee JW, Yeoh GP, Chan KW. Diagn Cytopathol. 2009 Apr;37(4):241-3. 33-False negative rate in cervical cytology. van der Graaf Y, Vooijs GP. J Clin Pathol. 1987 Apr;40(4):438-42. 34-Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T; Joint European Cohort Study. BMJ. 2008 Oct 13;337:a1754. 35-Long term duration of protective effect for HPV negative women: follow-up of primary HPV screening randomised controlled trial. Elfström KM,Smelov V, Johansson AL, Eklund C, Nauclér P, Arnheim-Dahlström L, Dillner J. BMJ. 2014 Jan 16;348:g130. 36-HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Ogilvie GS, Krajden M, van Niekerk D, Smith LW, Cook D, Ceballos K, Lee M, Gentile L, Gondara L, Elwood-Martin R, Peacock S, Stuart G, Franco EL, Coldman AJ. Int J Cancer. 2017 Jan 15;140(2):440-448. 37-Clinical human papillomavirus detection forecasts cervical cancer risk in women over 18 years of follow-up. Castle PE, Glass AG, Rush BB, Scott DR, Wentzensen N, Gage JC, Buckland J, Rydzak G, Lorincz AT, Wacholder S. J Clin Oncol. 2012 Sep 1;30(25):3044-50. 38-Five-year risks of CIN 3+ and cervical cancer among women withHPV-positive and HPV-negative high-grade Pap results. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, Cheung LC, Raine-Bennett T, Gage JC, Kinney WK. J Low Genit Tract Dis. 2013 Apr;17(5 Suppl 1):S50-5. 39-Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Lancet. 2001 Jun 9;357(9271):1831-6. 40-Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJ; International HPV screening working group. Lancet. 2014 Feb 8;383(9916):524-32. 41-Discrepant HPV/cytology cotesting results: Are there differences between cytology-negative versus HPV-negative cervical intraepithelial neoplasia? Tracht JM, Davis AD, Fasciano DN, Eltoum IA. Cancer Cytopathol. 2017 Oct;125(10):795-805. 42-Cervical cancer risk for women undergoing concurrenttesting for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Lancet Oncol. 2011 Jul;12(7):663-72. 43-Efficacy of cervical-smear collection devices: a systematic review and meta-analysis. Martin-Hirsch P, Lilford R, Jarvis G, Kitchener HC. Lancet. 1999 Nov 20;354(9192):1763-70. 44-A comparison of the three most common Papanicolaou smear collection techniques. Germain M, Heaton R, Erickson D, Henry M, Nash J, O’Connor D. Obstet Gynecol. 1994 Aug;84(2):168-73. 45-Liquid-based Papanicolaou smears without a transformation zone component: should clinicians worry? Baer A,Kiviat NB, Kulasingam S, Mao C, Kuypers J, Koutsky LA. Obstet Gynecol. 2002 Jun;99(6):1053-9. 46-Endocervical component in conventional cervical smears: influence on detection of squamous cytologic abnormalities. Ribeiro AA, Santos Sdo C, de Souza e Silva SR, Nascimento MA, Fonsechi-Carvasan GA, Carneiro MA, Rabelo-Santos M, Rabelo-Santos SH. Diagn Cytopathol. 2007 Apr;35(4):209-12.47-Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lew JB, Simms KT, Smith MA, Hall M, Kang YJ, Xu XM, Caruana M, Velentzis LS, Bessell T, Saville M, Hammond I, Canfell K. Lancet Public Health. 2017 Feb;2(2):e96-e107.
48-Risk of invasive cervical cancer in relation to management of abnormal Pap smear results. Silfverdal L, Kemetli L, Andrae B, Sparén P, Ryd W, Dillner J, Strander B, Törnberg S. Am J Obstet Gynecol. 2009 Aug;201(2):188.e1-7. 49-Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, Matchar DB. Ann Intern Med. 2000 May 16;132(10):810-9. 50-HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Ogilvie GS, Krajden M, van Niekerk D, Smith LW, Cook D, Ceballos K, Lee M, Gentile L, Gondara L, Elwood-Martin R, Peacock S, Stuart G, Franco EL, Coldman AJ. Int J Cancer. 2017 Jan 15;140(2):440-448. 51-Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. Cuschieri KS,Cubie HA, Whitley MW, Seagar AL, Arends MJ, Moore C, Gilkisson G, McGoogan E. J Clin Pathol. 2004 Jan;57(1):68-72. 52-Management of adolescents who have abnormal cytology and histology. Moscicki AB. Obstet Gynecol Clin North Am. 2008 Dec;35(4):633-43. 53-Minor Cytological Abnormalities and up to 7-Year Risk for Subsequent High-Grade Lesions by HPV Type. Maria Persson, K Miriam Elfström, Sven-Erik Olsson, Joakim Dillner, Sonia Andersson. PloS One. 2015 Jun 17;10(6):e0127444. 54-Adolescent cervical dysplasia: histologic evaluation, treatment, and outcomes. Moore K, Cofer A, Elliot L, Lanneau G, Walker J, Gold MA. Am J Obstet Gynecol. 2007 Aug;197(2):141.e1-6. 55-Type-specific prevalence and persistence of human papillomavirus in women in the United States who are referred for typing as a component of cervical cancer screening. Ralston Howe E, Li Z, McGlennen RC, Hellerstedt WL, Downs LS Jr. Am J Obstet Gynecol. 2009 Mar;200(3):245.e1-7. 56-Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Molano M, Van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, Meijer CJ, Muñoz N, Franceschi S; HPV Study Group. Am J Epidemiol. 2003 Sep 1;158(5):486-94. 57-Factors associated with type-specific persistence of high-risk human papillomavirus infection: A population-based study. Stensen S, Kjaer SK, Jensen SM, Frederiksen K, Junge J, Iftner T, Munk C. Int J Cancer. 2016 Jan 15;138(2):361-8. 58-Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. J Infect Dis. 2008 May 15;197(10):1436-47. 59-The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, Palefsky J. J Pediatr. 1998 Feb;132(2):277-84. 60-Chapter 5: Viral and host factors in human papillomavirus persistence and progression. Wang SS, Hildesheim A. J Natl Cancer Inst Monogr. 2003;(31):35-40.61-Natural history of cervicovaginal papillomavirus infection in young women. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. N Engl J Med. 1998 Feb 12;338(7):423-8.
62-Cervical cytology testing in teens. Moscicki AB. Curr Opin Obstet Gynecol. 2005 Oct;17(5):471-5. 63-Prospective evaluation of longitudinal changes in human papillomavirus genotype and phylogenetic clade associated with cervical disease progression. An HJ, Sung JM, Park AR, Song KJ, Lee YN, Kim YT, Cha YJ, Kang S, Cho NH. Gynecol Oncol. 2011 Feb;120(2):284-90. 64-Human papillomavirus infection is transient in young women: a population-based cohort study. Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. J Infect Dis. 1995 Apr;171(4):1026-30. 65-Natural history of cervical human papillomavirus lesions does not substantiate the biologic relevance of the Bethesda System. Syrjänen K, Kataja V, Yliskoski M, Chang F, Syrjänen S, Saarikoski S. Obstet Gynecol. 1992 May;79(5 ( Pt 1)):675-82. 66-Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Rositch AF,Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C, Smith JS. Int J Cancer. 2013 Sep 15;133(6):1271-85. 67-The natural history of human papillomavirus infection. de Sanjosé S, Brotons M, Pavón MA. Best Pract Res Clin Obstet Gynaecol. 2018 Feb;47:2-13. 68-Type-specific persistence of high-risk human papillomavirus infections in the New Independent States of the former Soviet Union Cohort Study. Kulmala SM, Shabalova IP, Petrovitchev N, Syrjänen KJ, Gyllensten UB, Johansson BC, Syrjänen SM; New Independent States of the former Soviet Union Cohort Study Group. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):17-22. 69-Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, Rozendaal L, Remmink AJ, Risse EK, van der Linden HC, Voorhorst FJ,Kenemans P, Meijer CJ. Lancet. 1999 Jul 3;354(9172):20-5. 70-Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. Rodríguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R; Proyecto Epidemiológico Guanacaste Group. J Natl Cancer Inst. 2008 Apr 2;100(7):513-7.71-Human papillomavirus persistence in young unscreened women, a prospective cohort study. Schmeink CE, Melchers WJ, Siebers AG, Quint WG, Massuger LF, Bekkers RL. PLoS One. 2011;6(11):e27937.
72-Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. Kjaer SK, van den Brule AJ, Paull G, Svare EI, Sherman ME, Thomsen BL, Suntum M, Bock JE, Poll PA, Meijer CJ. BMJ. 2002 Sep 14;325(7364):572. 73-Natural history of cervical intraepithelial neoplasia: a critical review. Ostör AG.Int J Gynecol Pathol. 1993 Apr;12(2):186-92. 74-Behavior of mild cervical dysplasia during long-term follow-up. Nasiell K, Roger V, Nasiell M. Obstet Gynecol. 1986 May;67(5):665-9. 75-Biologic course of cervical human papillomavirus infection. Nash JD, Burke TW, Hoskins WJ. Obstet Gynecol. 1987 Feb;69(2):160-2. 76-Cervical dysplasia in adolescents. Wright JD, Davila RM, Pinto KR, Merritt DF, Gibb RK, Rader JS, Mutch DG, Gao F, Powell MA. Obstet Gynecol. 2005 Jul;106(1):115-20. 77-Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Landy R, Pesola F, Castañón A, Sasieni P. Br J Cancer. 2016 Oct 25;115(9):1140-1146. 78-The association between cervical cancer screening and mortality from cervical cancer: a population based case-control study. Vicus D, Sutradhar R, Lu Y, Elit L, Kupets R, Paszat L; Investigators of the Ontario Cancer Screening Research Network. Gynecol Oncol. 2014 May;133(2):167-71. 79-Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. Andrae B, Kemetli L, Sparén P, Silfverdal L, Strander B, Ryd W, Dillner J, Törnberg S. J Natl Cancer Inst. 2008 May 7;100(9):622-9. 80-Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F.Lancet Glob Health. 2020 Feb;8(2):e191-e203. Polikistik Over
Polikistik Over